We can explore metathesis catalysts known as “Grubb catalyst” or “Schrock catalyst” to make insect pheromones.
The facile synthesis of a few lepidopteran insect pheromones can be accomplished using Ruthenium-based Z-selective olefin metathesis catalyst. Quite a few pheromones are approved by the EPA as pest control pheromone /semio-chemical agents.
These metathesis methods can be extended to synthesize other insect pheromone analogs containing cis-olefins in a similar manner and can be applied for EPA approval for pest control.
This metathesis chemistry can provide an economically feasible route to make several pheromones efficiently in good yields with high cis-selectivity in a minimal number of steps from commercially available olefins containing various functional groups such as alcohols, aldehydes, ketones, acetates, epoxides and unconjugated (E,Z) diene.
Terminal alkene can be reacted with seed oil derivatives in the presence of low catalyst loading (about 1 mol %) to yield the desired cross products in good yields with high cis-selectivity as an alternative to conventional methods used to form cis-olefins via cis-hydrogenation of alkynes and the Wittig reaction.
The synthesis of the pheromones can be taken up after understanding the reactivity and selectivity of catalysts shown below.
| Grubb Catalyst | Schrock Catalyst | Special Third Generation Catalyst |
|---|---|---|
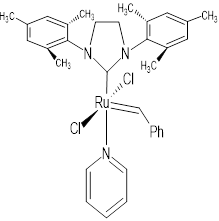 |
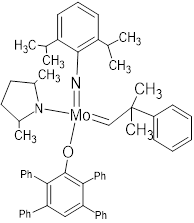 |
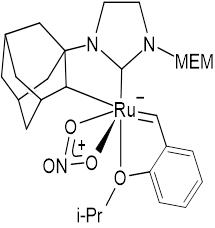 |
Some examples of shortest synthetic routes to cis-pheromones containing various functionality are shown below.
The reaction of 1-hexene with oleyl alcohol directly produced z,9-Tetradecenol in 77% yield (86% Z-isomer). The product z,11-Hexadecenol made from 11-eicosenol was obtained in 75% yield (86% Z-isomer), and can be oxidized using Swern method to yield pheromone z,11-Hexadecen-1-al. This synthesis is the shortest routes to form pheromones z,9-Tetradecenol and z,11-Hexadecen-1-al

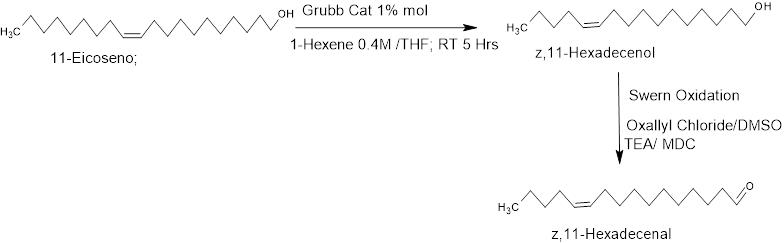
However, there is a limitation of this metathesis chemistry, for example, cross metathesis of the same seed oil derivatives with 1-butene is less efficient than with 1-hexene. Yield in case of 1-Butene is 40-50% (70-80% Z- isomer)
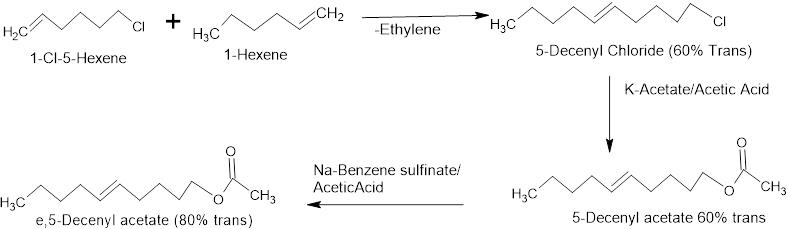
Metathesis coupled with isomerization can be designed to get trans isomer also. 1-Chloro-5-Hexene coupled with 1-Hexene using Grubb’s catalyst gives 5-Decenyl chloride and ethylene gas. It is the reacted with Potassium acetate to give 5-Decenyl Acetate (60:40:: E:Z). This can be further isomerized using Sodium Benzene sulfinate to give 80% trans isomer with overall yield of 25-30%.
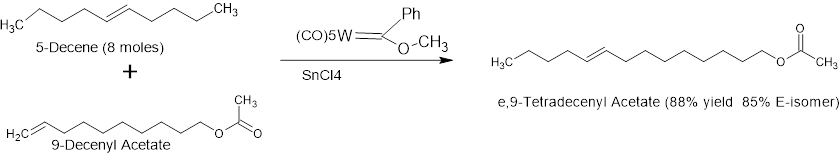
Similarly, e,9-Tetradecenyl acetate can be made as follows: Metathesis between 9-Decenyl acetate and 5-Decene using early generation metathesis catalyst Phenyl Methoxy pentacarbonyl Tungsten & Tin tetrachloride
We can deliver high value insect pheromones using these alcohol-substituted starting plant oil materials via metathesis.
Please contact ChemHub for more further information.









