There are some products can be made starting from Vanillin. First, let us understand what is Vanillin?
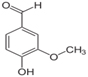
Vanillin based products is a quite prolific molecule. It is phenolic aldehyde containing two more functional groups, ether and hydroxyl groups.
It can be natural and is generally extracted from Vanilla bean. The natural vanillin is generally called Vanilla and contains the ethoxy analog as well and has very strong note. But, natural vanillin is quite expensive, nearly >15 times than synthetic analog. And it is generally used as flavoring agent in food.
Synthetic Vanillin is now more used to for F&F application and also can used to make other other derivatives for F&F application and also in pharmaceuticals.
There are two types of Vanillin:
Plain Vanillin having methoxy group ((−O−CH3))
CAS No: 121-33-5
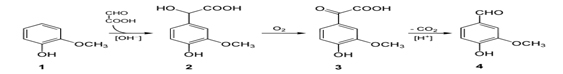
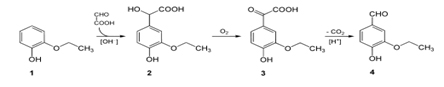
Scheme for making Vanillin: Chemical synthesis of Vanillin is a two-step process, in which guaiacol (1) reacts with glyoxylic acid by electrophilic aromatic substitution. The intermediate formed, vanillylmandelic acid (2) is then converted to 4-Hydroxy-3-methoxyphenylglyoxylic acid (3) and finally followed through Vanillin (4) by oxidative decarboxylation.
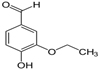
Ethyl Vanillin having ethoxy group (−O−CH2CH3)
CAS No: 121-32-4
Scheme for making Ethylvanillin: Ethylvanillin is prepared from catechol, beginning with ethylation to give guaethol (1). This ether condenses with glyoxylic acid to give the corresponding mandelic acid derivative (2), which by oxidation (3) and decarboxylation, gives ethylvanillin (4)
Ethyl Vanillin has a stronger note and is more expensive than plain Vanillin.
In this blog, Plain synthetic vanillin will be described. There are a few global producers for Synthetic Vanillin. The first commercial synthesis of vanillin began with natural compound Eugenol, 4-allyl-2-methoxyphenol. Today, artificial vanillin is made either from Guaiacol. The scheme is given below:
Not too long ago, biosynthetic Vanillin was developed, but it became very expensive and is not feasible to make other derivatives economically. The best cost, competitive Vanillin is made from Petrochemical waste Lignin or Guaiacol.
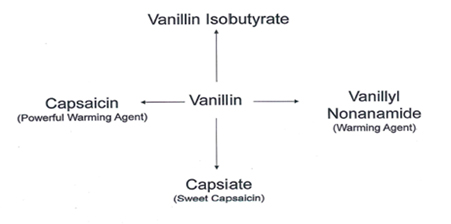
Vanillin has been used as a chemical intermediate in the production of pharmaceuticals, cosmetics, and fine chemicals. Most of vanillin production was used in the synthesis of specialty chemicals. In this blog, four different types of specialty products are described having application in F&F industry.
A general flow chart of four different products can be made from Vanillin that are of importance in Flavor & Fragrance industry
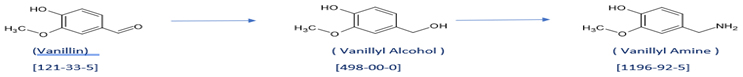
At ChemHub, we have developed the process to make two key intermediates starting from Vanillin:
Vanillyl alcohol (CAS No: 498-00-0) and Vanillyl amine (CAS No: 1196-92-5).
These two key intermediates are used to make final products. Schemes are shown below. The last scheme is the preparation of Vanillin Isobutyrate directly from Vanillin.
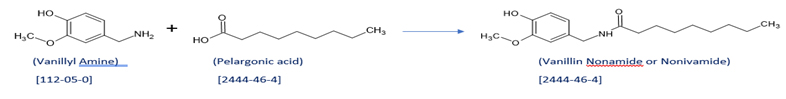
- Scheme for making Nonivamide:
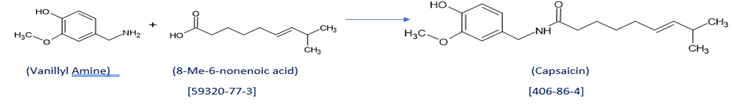
- Scheme for making Capsaicin:
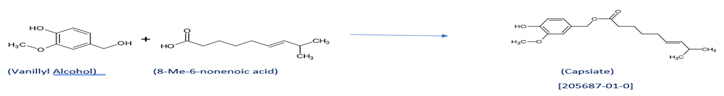
- Scheme for making sweet Capsaicin (Capsiate):
- Scheme for making Vanillin IsoButyrate (VIB):

Nonivamide:
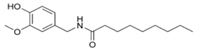
Vanillin Nonamide is also known as Nonivamide. Nonivamide can also be called pelargonic acid vanillylamide or PAVA.
CAS Number: 2444-46-4
It is in capsaicinoid category. It is manufactured synthetically from Vanillylamine and pelargonic quite cheaper than capsaicin.
Nonivamide is used to add pungency in seasonings and spice blends. It is used in the bakery industry to induce a hot sensation.
and is also used in the pharmaceutical industry as a cheaper alternative to capsaicin.
It can also deter animals, such as deer and squirrel from consuming seeds and crops. It is used in pepper spray irritant for self-defense purpose and can be used in dispersing peaceful demonstration or riot control. It can also be used as lethal in the chemical warfare.
Capsaicin:
CAS Number: 404-86-4
Capsaicin (8-methyl-N-vanillyl-6-nonenamide) is an active component of chili peppers, which can be synthesized as well. It is a chemical irritant for mammals, including humans, and produces a sensation of burning in any tissue with which it comes into contact. Capsaicin and several related are called capsaicinoids having use as deterrents against certain mammals. Pure capsaicin is a hydrophobic, colorless, highly pungent, crystalline solid compound. it is also commonly used in food products to provide added spice and heat. In high concentrations, capsaicin will also cause a burning effect on other sensitive areas, such as skin or eyes. The degree of heat found within a food is often measured on the Scoville scale.
There has long been a demand for capsaicin-spiced products such as Tabasco sauce and Mexican salsa. It is reported to experience pleasurable and even euphoric effects from capsaicin via endorphins release. It also has application for relief of minor aches and pains of muscles and joints associated with arthritis, backache, and sprains,
Capsaicin is being used to reduce the symptoms of peripheral neuropathy, providing pain relief from, HIV-neuropathy, and diabetic neuropathy., Capsaicin is shown to decrease LDL cholesterol levels.
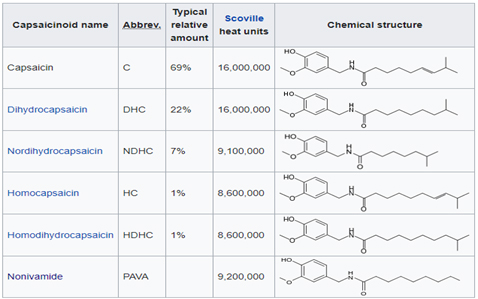
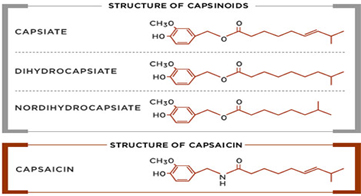
There are six natural capsaicinoids shown below. Only, Vanillylamide or Nonivamide (PAVA) and Capsaicin are produced synthetically for most applications
Sweet Capsaicin (Capsiate):
Major capsinoids in nature:
Capsiate ( 4-hydroxy-3-methoxybenzyl (E)-8-methyl-6-nonenoate), (CAS No. 205687-01-0)
Dihydrocapsiate (4-hydroxy-3-methoxybenzyl 8-methylnonanoate), (CAS No. 205687-03-2)
Nordihydrocapsiate (4-hydroxy-3-methoxybenzyl 7-methyloctanoate), (CAS No. 220012-53-3)
Capsinoids category include capsiate, dihydrocapsiate, and nordihydrocapsiate, and naturally present in chilli. Even though, structurally similar to Capsaicin, the biggest difference is in Scoville Heat unit (SHU) where the Capsinoids have an estimated 1/1000 that of capsaicin. This is the main reason, the Capsiate is dubbed as “ Sweet Capsaicin”. Many health effects have been reported through scientific study, including anticancer, anti-inflammatory, and analgesic activities, and weight management.
Structural differences between Capsaicin and Capsinoid family of compounds are illustrated below. Capsinoids have an ester bond in their structures, whereas Capsaicin has characteristic amide bond.
Capsaicin vs. Capsinoids? Which one to chose
Both are shown have same effect except the taste and SHU. The real difference is Capsaicin is more effective to deter animals, where larger amount of Capsiate can be absorbed in the body without burning effect. There are many ongoing investigation for comparison and other benefits.
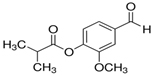
Vanillin IsoButyrate (VIB):
CAS No: 20665-85-4
Very little information is available at this moment.ChemHub is making it,
Please contact us at ChemHub, Inc. Sayreville, NJ, US. Phone: 908-548-0790
Email: info@chemhub.com
Website: www.chemhub.com